This past June, during a debate in the Senate Health, Education, Labor and Pensions (HELP) Committee, Senator Richard Burr (R-NC), the committee’s Ranking Member, demonstrated that he does not understand very important developments in our nation’s approach to drug regulation and safety. It was almost as if Sen. Burr had fallen asleep over 20 years ago and woke up believing that nothing had changed in the world of drug regulation. Additionally, in his opposition to Senator Bernie Sanders’s forward-thinking, bi-partisan drug importation amendment to the FDA Drug User Fee Bill, Sen. Burr insulted Italy!
Why am I writing about this now? Last Friday, an article I wrote relating to that June HELP committee meeting was published in The Hill. There, I talk about an unwanted and unnecessary rider to the FDA Drug User Fee Act that would undermine prescription drug importation. But there’s a story behind that story: Congressional committee meetings sometimes feature members who literally don’t know what they are talking about, and I felt the need to discuss it.
Sen. Sanders raised the issue that high drug prices are the problem Americans care about most— that egregious pricing is quite literally killing them. Those are facts. He said we must reform our regulations to help them find relief, including using drug importation. I agree with him. On the other hand, honest and informed people can disagree, but that’s not what happened here. Sen. Burr responded, saying: “You want to kill this bill? Do importation… I hear the laugh, Bernie, you’re entitled to your own opinion, but you’re not entitled to your own set of facts.” Burr went on to show that he did not know very key facts related to the subject matter. Senator Burr was either honest and uninformed, or dishonest and informed.
Senator Sanders’s amendment, which would have substantially widened the scope of drug importation from Canada, added the United Kingdom as a permissible country from which to import, and allowed for additional countries to be added in the future. Senator Burr’s main argument against drug importation was that America cannot trust countries in the European Union – or anywhere else for that matter – with drug safety. His essential focus was that the EU does not have the same strong pharmaceutical regulations as the U.S. That’s just plain wrong. The European Union’s drug regulatory authority is comparable, if not arguably superior, to that of the United States.[LM1]
Burr recounted how, in the late 1990s, FDA personnel went to Europe to see if the U.S. could harmonize drug regulatory standards with the EU. The Clinton administration found that they could not do so. Burr explained that EU countries did not have one standard, but rather accepted the standards of each member-state. He stated, “…so they accept whatever safety standard from an EU member applies. That means within the EU it may have been an Italian drug that was approved by a much lower standard in Italy but everybody in Europe takes it.”
“Unless something’s changed on that,” he went on, “then I would accept that if we had a determination today, we would probably find the same thing.”
Burr implies that, in the year 2022, we cannot trust the EU’s drug regulatory system because we could not trust it during the Clinton administration… over twenty years ago.
In 2019, years ago still, the highly-respected European Medicines Agency, with recognition by the FDA, announced that the EU and US reached “a milestone in mutual recognition of inspections of medicines manufacturers.”
Still don’t trust the Europeans? From the FDA’s website: “FDA has MRAs [mutual recognition agreements] in place with the EU and the United Kingdom, respectively.” In other words, today, the U.S. recognizes that all EU member states – including Italy – have comparable capabilities to regulate the manufacture of pharmaceutical drugs and no one brought that up during the committee meeting.
Want more proof? The FDA’s own data shows that the EU has stronger drug safety outcomes than the U.S. when it comes to Current Good Manufacturing Practices. In fact, the U.S. performance was slightly lower than the world at large. See FDA’s data below.
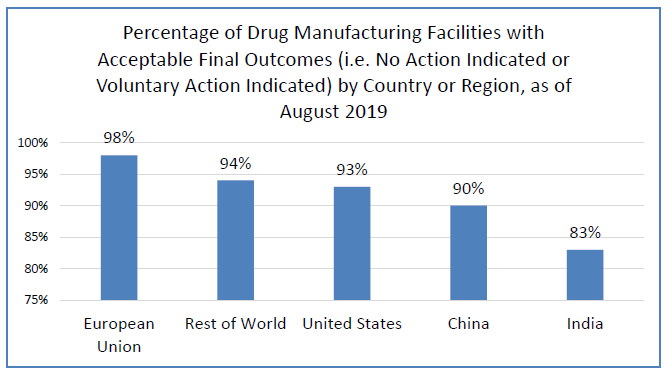
I’m not taking anything that Sen. Burr said out of context. But, in case I am, to fact-check me, please feel free to watch Burr’s entire presentation.
Members of Congressional committees are supposed to have specialized and up-to-date knowledge on matters of great national importance that fall within a committee’s jurisdiction. To that end, every five years, the HELP committee, which has jurisdiction over pharmaceutical regulations, plays a role in drafting FDA user fee reauthorization legislation. The act sets new fees and rules related to drug manufacturers who apply to the FDA when seeking approval of a new drug. It also usually includes other policies meant to strengthen our regulatory system and improve access to prescription drugs. To that end, committee leaders should not be able to get away with being ignorant of the subject matter at hand. It leads to bad public policy outcomes for Americans and potential friction with allies, such as Italy!
